First Analytics-Supported Opioid Addiction Study Receives FDA Fast Track Breakthrough Designation
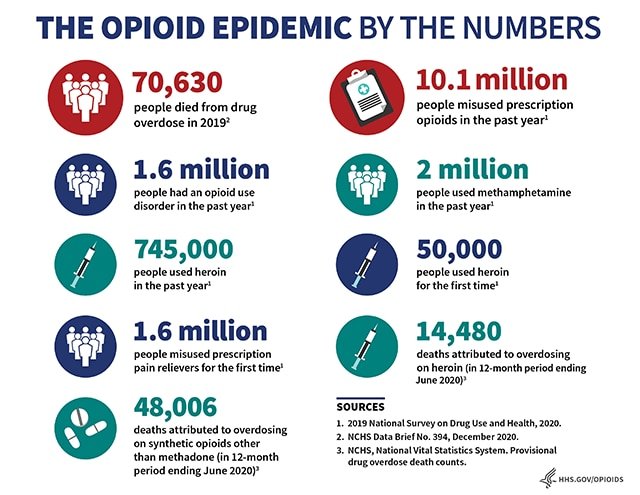 The opioid epidemic has taken a toll, as shown by this infographic produced by the U.S. Department of Health and Human Services. The misuse of opioids was declared a public health emergency in 2017.
The opioid epidemic has taken a toll, as shown by this infographic produced by the U.S. Department of Health and Human Services. The misuse of opioids was declared a public health emergency in 2017.
In 2020 a research team studying a new novel treatment approached First Analytics for statistical analysis support. They had initial funding from the National Institute of Drug Abuse (NIDA). Our work together is described in this case study.
Our statistical analysis showed unequivocally that the treatment produced a significant reduction in opioid cravings beyond the placebo, averaging nearly 12 points better on the Opioid Craving Scale, and that it was effective for patients of different genders, ethnicities, and drug use backgrounds. More details can be found in this published paper.
On September 2, 2022, the Federal Drug Administration announced that the research team would receive so-called fast track breakthrough designation, along with a much larger grant from NIDA for a larger, 2-year study. The FDA defines fast track breakthrough designation as a process designed to expedite the development and review of drugs which may demonstrate substantial improvement over available therapy.
The First Analytics team has range, using analytics and machine learning to address a wide variety of business problems. But our work with analytics in other areas of public good or sustainability, such as safety, energy conservation, and pandemic response, are among the most personally rewarding things we do.